Predict the Molecular Geometry About O in the Molecule Of2.
If the molecular weight is 223 amu what is the molecular. Thus there are 20 valence electrons available for OF 2 that help the atoms to form bonds.

Of2 Lewis Structure Molecular Or Electron Geometry Polar Or Non Polar
The total valence electron present in OF2 is 20.
. A Draw the Lewis electron structure of the molecule or polyatomic ion. Predict the shape of the molecule HCN. You can figure this out by doing the Lewis Structure.
It is helpful if you. The first and foremost step is to calculate the total number of valence electrons in an OF2 molecule. 6 7 x 2 20 valence electrons.
5-1 kcalmol much smaller than covalent bonds with energies of 50-200 kcalmol. E follow the preceding procedure for constructing the VSEPR Model. It has two atoms connected to carbon and no lone pairs on the carbon cent.
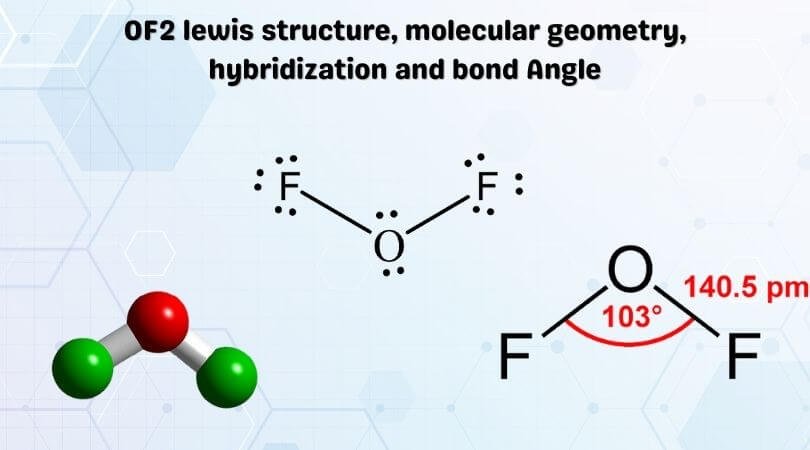
What theory is the assumption that molecular shapes are largely determined by the repulsion of regions of electron concentration. Oxygen difluoride OF 2 isnt too tough of a Lewis structure since it only has single bonds. Oxygen belongs to group 16 the chalcogen family and has a valency of 6.
There are three atoms bonded to this C atom and no nonbonding pairs and so it has three electron domains about it. Cl2 H2O HCN NH 3 CO 2 H 2 O 2 O 3 SO 4 2 o Use the VSEPR theory to predict the molecular geometry of each of the following. Drawing the Lewis Structure for OF 2.
To predict the O CC bond angle we must examine the leftmost C atom which is the central atom for this angle. According to the Lewis formula the central atom Xe forms 3 double bonds with O-atoms and 2. The hybridization of OF2 is Sp³.
Fluorine belongs to the family of halogen in group 17 and has a valency of 7. W the Lewis structure of water. There are four electron pairs around the central atom.
B What accounts for the observed difference in the properties of ionic and molecular compounds. Predict the molecular geometry of water H 2 O. Therefore it creates some dipole moment in the O-F bond which causes OF2 to be polar in.
Which of the. PF 5 phosphorus pentafluoride a catalyst used in certain organic reactions H 3 0 hydronium ion Given. What is the angle of the bonds in a pure tetrahedral arrangement.
Predict the shape of the molecule H2CO. Oxygen difluoride is polar in nature. The electron geometry of OF2 is tetrahedral and molecular geometry is Bent.
Therefore the total number of valence electrons 6 72 20. So youll be busy drawing some dots. The lone pair of electrons is at the top of the SO2 molecule.
SO2 a Bent molecular shape. Two bond pairs an Tables 2 to predict the geometry of the molecule. Since the molecular geometry shape of OF2 is bent that results in two dipole moments between OF2 atoms which dont get canceled out with each other because of bent shape.
The 2s and 2p orbitals of the Oxygen atom are hybridized which means there is a formation of four hybridized orbitals. T the number of electron pairs around the central atom. So this yourselves Kenya and this just uh trying gonna play now as this is This looks I mean you always look at shit concentrating the long pair of electrons.
Two-hybrid orbitals contain lone pairs. So in this question better to give the molecular geometry huff each our structure. A molecular compound is composed of 588 Xe 72 O and 340 F by mass.
Hence the molecular formula of the compound is eqrm XeO_3Cl_2 eq. A quick explanation of the molecular geometry of OF2 including a description of the OF2 bond anglesLooking at the OF2 Lewis structure we can see that there. Try to draw the OF 2 Lewis structure before watching the video.
CO2 is a linear molecule. The predicted electron-domain geometry is trigonal planar resulting in an ideal bond angle of 120. Oxygen difluoride F2O lewis structure contains 4 nonbonding electrons and 4 bonding electrons.
Their electronegativity difference is 054 which indicates that the oxygen difluoride compound is polar. There are 20 valence electrons available for the Lewis structure for OF 2. 2s 2px 2py and 2pz.
Using the VSEPR model predict the molecular geometry of each molecule or ion. So the molecule will become sp3 hybridized. ICl 3 SO 2 H 2 Se and NH 4.
O undergoes sp 3 hybridization. The SO2 bond angle will be 120 degrees since it has a Bent molecular geometry. Count number of lone pairs on central atom and number of atoms bonded to the central atom.
Use VSEPR to predict the geometry of the molecule.

Solved Part A Predict The Molecular Geometry About O In The Chegg Com
Of2 Lewis Structure Molecular Geometry Hybridization And Bond Angle Geometry Of Molecules
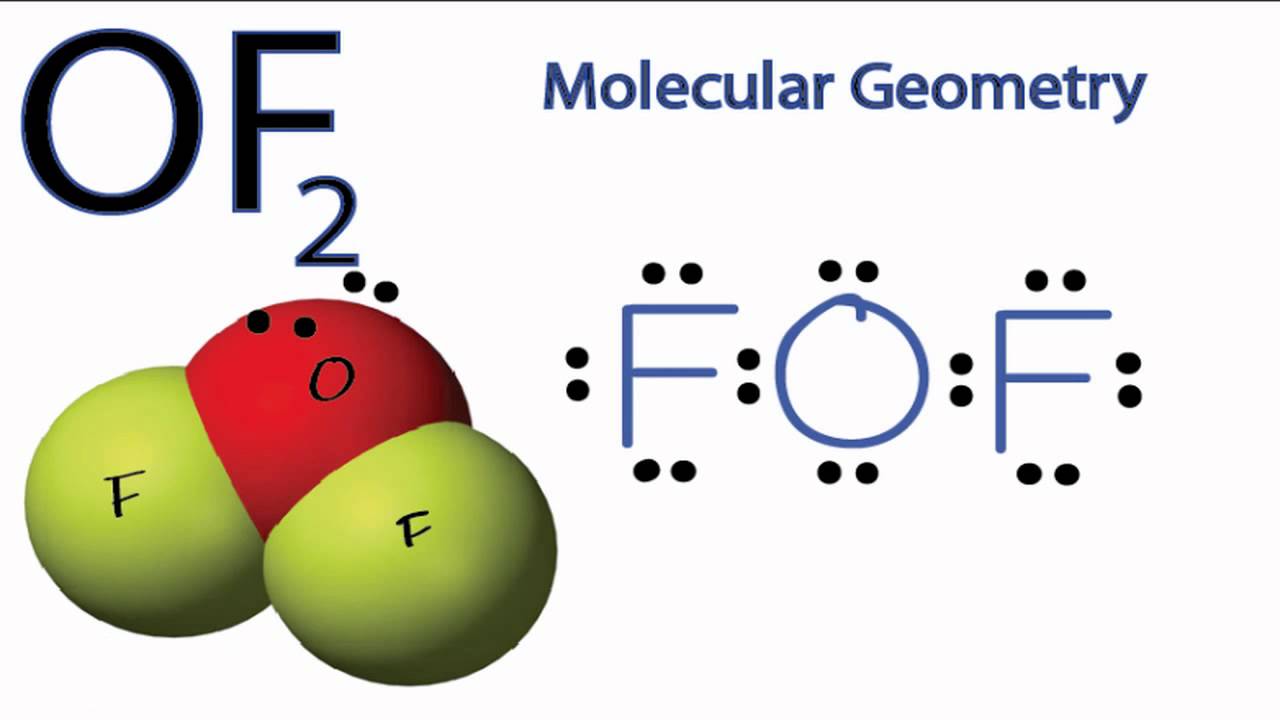
Of2 Molecular Geometry Note Precise Bond Angle Is 103 1 Youtube
Comments
Post a Comment